SCFAs Part 5: The propionate-autism connection
The prevalence of autism and other neuropsychiatric diseases has grown tremendously in recent decades. Environmental factors are sure to play a role, including the gut microbiota and its all-too important metabolites, short-chain fatty acids (SCFAs).
So far in the SCFA series, I’ve discussed the benefits of butyrate, the harms of excess butyrate, and the role of acetate in metabolic syndrome and obesity. In this article, I’ll talk about propionate, and its emerging connections to autism and other mitochondrial-related, neurological, and neurodevelopmental diseases. Don’t know what a SCFA is? Be sure to read my brief intro to SCFAs first!
The dramatic rise in autism spectrum disorders
When autism was first reported in the mid-20th century, it affected 1 in 10,000 people. Today, autism spectrum disorder (ASD) affects 1 in 68 people in the United States. ASD is characterized by social and communication impairments, sensory abnormalities, and repetitive behavior.1
Yet evidence increasingly suggests that ASD is not a brain disorder, but a “whole body” disorder. Those with ASD present with systemic abnormalities in metabolic and immune function,2,3 gastrointestinal symptoms, restrictive eating, and seizure disorders.4,5
Genes involved in mitochondrial function, immune regulation, and neural circuit formation have been linked to autism,6 but known genetic factors only account for 10-20% of ASDs. Some children even develop normally before regressing into ASD. This has especially been observed in populations that have migrated from underdeveloped countries to developed ones.7 Every Somalian population that has emigrated to Western countries has noticed an increased prevalence of ASD in their community. They call it the “Western Disease” since they don’t acquire it in their homeland of East Africa.
This means that environmental factors play a major role, and that autism arises from the complex interaction of genes with the environment. Recent evidence suggests that gut microbial metabolites, and, in particular, the SCFA propionate, may play a role. But before we dive into propionate in autism, let’s cover the roles that propionate plays in the body.
Too much of a good SCFA?
In part 3 of the SCFA series, we saw how four-carbon butyrate, a potent anti-inflammatory and crucial molecule for maintaining gut barrier function, could potentially be harmful in high concentrations, especially in the context of mucosal inflammation. Interestingly, a similar paradox may hold true for three-carbon propionate, where low concentrations have benefits, but high concentrations are toxic.
Propionate has wide-reaching effects on physiology. In the gut, propionate stimulates smooth muscle contractions,8 increases mucus secretion,9,10 promotes antimicrobial peptide expression, dilates colonic arteries11, and increases release of serotonin from gut endocrine cells.12 Propionate also influences intracellular pH, mitochondrial function, lipid metabolism, neurotransmitter synthesis and release, immune function, and gene expression.13 Propionate has been suggested to have weight loss, anti-inflammatory and cholesterol-lowering properties.14,15 In mice, propionate has been shown to induce the production of satiety hormones in the gut to reduce food intake and protect against diet-induced obesity16,17. A recent study found that administration of the short-chain fatty acid propionate significantly attenuated spondyloarthritis, a HLA-B27-associated inflammatory disease.17
However, excess propionate can be problematic. In propionic acidemia, a genetic error of metabolism, accumulation of propionate is associated with acidosis, developmental delay, seizure, increased oxidative stress, mitochondrial dysfunction, and bouts of gastrointestinal symptoms.18 Excess propionate has also been reported in irritable bowel syndrome19, and of course, autism.
A link between propionate and autism: animal models
Researcher Dr. Derek MacFabe is at the forefront of microbiome-related research and is largely responsible for elucidating the propionate-autism connection. His lab discovered that excess propionate is capable of inducing “behavioral and brain effects remarkably consistent with findings in persons with ASD”.20
In one study, they injected propionate directly into the cerebrospinal fluid of the rat’s brains. Within just 2-30 minutes, propionate-treated rats showed repetitive behaviors, increased hyperactivity, impaired social behavior, and evidence of seizure activity, compared to those receiving placebo infusion. While butyrate- and acetate-treated rats did show some abnormalities, propionate elicited the strongest ASD-like behaviors.21
In another study, they looked at the effects of systemic propionate by peripherally injecting pregnant rats and then their offspring. Propionate increased anxiety-like behavior and repetitive behavior, particularly in rats that were exposed both pre- and postnatally.22
Evidence for a propionate-autism link in humans
This link is potential, but still unproven in humans. As we saw with acetate, we can’t take be sure that what occurs in animal models also occurs in humans. In a recent review paper, Dr. MacFabe cautioned:
“The evidence of many effects of [propionate] on diverse biological pathways being consistent with findings with ASD patients is intriguing but largely correlative. Novel translational models […] coupled with longitudinal human studies are necessary to correlate these biomarkers to autistic regression or clinical improvement.” 20
We also don’t know whether an altered gut microbiota is the cause of ASD-related symptoms, or simply a consequence of the same underlying pathophysiological features of autism.
Still, it’s well accepted that birth by Cesarean section, early infections, and antibiotic exposure all may alter the developing gut microbiota, and are risk factors for ASD.23 Moreover, children with ASD have also been shown to have increased levels of fecal propionate,24 and ASD-associated bacteria include several known propionate-producing bacteria: Clostridia, Bacteroides, and Desulfovibrio.25
Several recent clinical studies have also found elevated urinary markers of Clostridia metabolites and altered carbohydrate metabolism in GI-tract biopsies in ASD patients,26 and others have reported temporary behavioral improvements following treatment with antibiotics that knockdown levels of propionate-producing bacteria.27
Despite the need for more human studies, the mechanisms at play in animal models also seem plausible in humans. We’ll dive into those in the next section.
The mechanisms: how propionate may contribute to autism
Fair warning: this section is full of biochemistry and complicated terminology. It’s not crucial, so if you get bogged down in the details, I’ll provide a summary at the end of the article.
Gap junction function
Dr. MacFabe postulates that many of the effects of propionate may be due to its ability to close gap junctions.28 Gap junctions connect the cytoplasm of adjacent cells and allow the passage of small molecules and ions between them. They are vital for synchronizing neural electrical activity, and play crucial roles in early brain development. A decrease in gap junction coupling may also inhibit cortical pruning, a phenomenon consistent with the increased density of neurons found in ASD patients.21
Furthermore, gap junction “knockout” mice (mice are genetically engineered to not express any gap junction proteins), have abnormal brain development and behaviors, seizure disorders, and exaggerated responses to neurotoxic insults.29
Mitochondrial dysfunction
ASD often occurs with genetic and biochemical changes that are consistent with mitochondrial dysfunction.30 Evidence suggests that propionate may be responsible for these changes by interfering with the mitochondrial TCA cycle.
The mitochondrial tricarboxylic acid (TCA) cycle is a key step in cellular energy production. The products of the TCA cycle are NADH and FADH2, which carry high energy electrons to the electron transport chain (ETC), where their reducing power is used to produce ATP (cellular energy). You can see from the diagram below that one round of the TCA cycle produces 3 molecules of NADH and 1 molecule of FADH2. Propionate enters the TCA cycle via conversion to succinyl CoA. In small amounts, this helps to maintain TCA cycle intermediates, and is beneficial to cellular energy production.

However, substantial amounts of propionate entering the TCA cycle bypasses the first four TCA enzymes and may cause a shift in the cycle. Flux increases through the latter half of the cycle, producing a buildup of citrate, while the proximal half becomes “backed up” due to feedback inhibition by succinyl CoA.

This has several consequences. First, this alters the ratio of NADH: FADH2, resulting in 1:1 production instead of 3:1. When these energy carriers reach the electron transport chain, NADH feeds into ETC Complex I and FADH2 feeds into Complex II.

Each NADH molecule results in the production of 3 molecules of ATP, while each FADH2 molecule results in the production of 2 molecules of ATP. Thus, a propionate-induced shift in TCA cycle flux will result in less NADH production, and a deficiency of energy carriers at Complex I, leading to less overall ATP formation. Indeed, children with ASD have been shown to have a deficiency in ETC complex 1.
Secondly, the buildup of mitochondrial citrate will result in citrate being transported into the cell cytosol. Citrate inhibits phosphofructokinase, the key regulatory step in glycolysis. Citrate also increases the formation of malonyl CoA, which inhibits CPT-1, the transporter that shuttles fatty acids into the mitochondria. This effectively blocks fatty acid oxidation.
Context matters: paradoxical propionate
To learn more about propionate’s role in mitochondrial dysfunction, students in Dr. McFabe’s laboratory cultured immune cells from ASD and control patients with various concentrations of propionate with and without reactive oxygen species (ROS). Paradoxically, they found that propionate improved mitochondrial function if ROS were not present; however, it negatively impacted mitochondrial function in the presence of ROS. In other words: propionate “can have both beneficial and toxic effects on mitochondrial function, depending on concentration, exposure duration, and microenvironment”.31
As a weak acid, propionate uptake increases under conditions of intracellular acidification and can become more concentrated within cells.32 Ongoing work by Dr. Sydney Finegold has identified bacterial populations like Desulfovibrio, which produce propionic acid and hydrogen sulfide. The presence of hydrogen sulfide may increase propionate’s ability to damage mitochondrial function33, possibly by acidifying cells.
The carnitine connection
Impairments in carnitine metabolism may also play a role in neurodevelopmental disorders such as ASD. Carnitine is most well-known for its involvement in fatty acid β-oxidation. Fatty acids cannot freely cross the mitochondrial membrane to be metabolized, and must instead be conjugated to the molecule carnitine for transport across the mitochondrial membrane. However, carnitines also play a role in lipid synthesis, cholinergic neurotransmission, membrane stability, and antioxidant activity. Carnitine is therefore important for cellular energy, brain development, and brain function.34
Many patients with ASD have a relative carnitine deficiency, which may be due to both inherited and acquired factors.35 For example, certain antibiotics are known to reduce carnitine levels.36 Oral carnitine and its derivative acetyl-L-carnitine have been shown to be neuroprotective and have promise as therapeutic agents in ASD and other neurodevelopmental disorders.
An epithelial energy crisis
If carnitine is deficient and a buildup of citrate is preventing fatty acids from being transported into the cell, brain cells aren’t the only ones that will suffer. Gut epithelial cells rely on fatty acid oxidation of butyrate for 70% of their cellular energy, and metabolism of this SCFA helps maintain gut integrity. Without fatty acid oxidation, the gut will become permeable.
Indeed, 36% of autistic patients have measurably increased intestinal permeability, and almost half have gastrointestinal symptoms of some sort.37
Can we test for propionate?
Of course, parents will be asking, can we get our child tested for propionate? Yes and no. There are several tests that might point to excess propionate, many of which I’ve already mentioned in some capacity:
- Fecal SCFA testing: measures levels of the SCFA propionate in stool
- Fecal microbiota testing: measures potential propionate-producing bacterial genera in the gut microbiota (Clostridia, Bacteroidetes, etc.)
- Urine Organic Acids Test Clostridia metabolites
- Urine Organic Acids Test TCA cycle metabolites: assessment of the ratio of distal half TCA metabolites to proximal half metabolites.
However, each of these has limitations. Propionate has a habit of hiding inside cells, which makes it difficult to detect, even in patients with a metabolic crisis. However, if several of the markers above are present, you can be more confident that you are dealing with propionate-induced or propionate-exacerbated ASD.
Strategies for modulating propionate production
So, let’s say propionate is causing dysfunction and exacerbating symptoms. What can we do? While most treatments are still empirical and based on anecdotal evidence, there are several ways to potentially reduce gastrointestinal propionate production and cellular levels of propionate.
Antibiotics
Metronidazole and vancomycin have been used in patients with ASD with some success. These antibiotics have broad-spectrum activity against gram-positive bacteria, including propionate-producing members of the Clostridium genus. Of the two, vancomycin is believed to be safer, since under normal circumstances, oral vancomycin is not significantly absorbed into circulation, while metronidazole is systemically absorbed and may have adverse systemic side effects.38
However, the effects of either antibiotic on propionate production may be short-lived. A small, 8 week, partially blinded clinical trial found that vancomycin was temporarily effective for treating ASD in 8 of 11 children, but the benefits didn’t last long.27 Clostridia quickly reappeared after cessation of antibiotic treatment, likely due to their spore-forming properties. Thus, antibiotics alone are not sufficient to knock down propionate production in the long-term.
Restoring gastrointestinal pH and SCFA ratios
Altering the pH of the gut has major consequences for gut microbiota composition. Studies of human fecal microbial communities found that at pH 5.5, beneficial butyrate-producing bacteria comprised 20% of the total population. When the pH rose to 6.5, these bacteria almost completely disappeared, and acetate- and propionate-producing bacteria became dominant.39 Of course, restoring colonic pH in a patient with ASD is no easy task, and is not a currently feasible treatment option.
On the other hand, butyrate has shown some promise for the treatment of ASD and other neurological disorders,40 though the mechanism is not completely understood. Of course, butyrate improves barrier function, regulates the immune system, and may help to reduce the pH in the gut, selecting against propionate-producers. Competition of butyrate for transporters may also reduce the amount of propionate absorbed from the gut into circulation. More research is necessary to determine how SCFA ratios may impact ASD.
Correcting nutrient deficiencies
Biotin and vitamin B12 are essential cofactors for the enzymes that break down propionate and allow it to enter the TCA cycle. Dietary deficiencies of these vitamins may further impair propionate and carnitine metabolism and contribute to mitochondrial dysfunction.
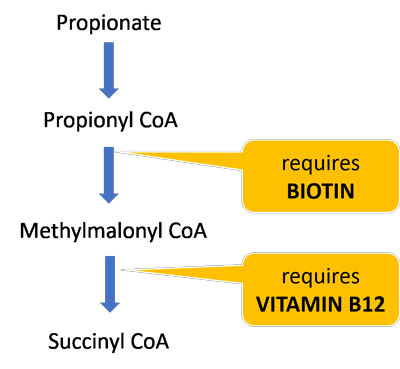
About half of biotin is produced by the gut microbiota, so those with gut dysbiosis are particularly susceptible to deficiency. One study found that children with ASD had significantly lower biotin levels compared to controls.41 A follow-up study found that replenishing biotin levels was highly correlated with ASD symptom improvement,42 suggesting that many ASD patients may benefit from biotin supplements.
Gluten-free, Casein-free Diet
In the last decade, gluten-free, casein-free (GFCF) diets have become an increasingly popular treatment for patients with ASD. While removing these immunogenic proteins from the diet may have independent benefits, GFCF diets may also have implications for propionate production.
Notably, propionate is a major animal silage and food preservative in wheat and dairy products as sodium propionate or calcium propionate salts, and occurs naturally in some dairy products like Swiss cheese.43,44
Propionate is also produced directly or indirectly by many ASD-associated bacteria, such as Clostridia and Desulfovibrio, from the fermentation of refined and wheat-based sugars.45,46 In other words, when you feed these bacteria certain grains and refined carbohydrates, they start making more propionate.
Removing these foods may have benefits on propionate metabolism and for gut barrier function. The same study mentioned above that found increased intestinal permeability in children with autism found that those on a GFCF diet had lower intestinal permeability scores than controls!37
Other dietary sources of propionate
Beyond wheat and dairy, propionate is also added to many refined foods as a preservative. In fact, the food industry and agriculture are increasingly using propionate and related chemical derivatives.47 Nitropropionate is a derivative of propionate produced by many plants and fungi. It’s a potential contaminant of sugar cane, soy sauce, and processed rice, and a potent mitochondrial and neurotoxin.48
Other foods stimulate propionate production. Artificial sweeteners like aspartame and saccharin, for example, has been shown to dramatically increase gut propionate levels in rodents.49,50 A 2005 study found that infants fed formula had 2.5 fold greater propionate levels than breastmilk-fed infants. Adding oligosaccharides to the formula only partially ameliorated the excess propionate production.51
The GAPS Diet and carbohydrates
Dr. Natasha Campbell-McBride is well known among the autism community for her book Gut and Psychology Syndrome (GAPS). Dr. McBride believes that neuropsychiatric disorders, including autism, arise in the gut. She has helped numerous children and adults reverse autistic behavior and regain normal daily function using a modified version of the Specific Carbohydrate Diet (SCD) called the GAPS Diet.
The GAPS Diet removes all fermentable carbohydrates from the diet for a period of time, allowing inflammation to subside and the gut barrier to heal and seal. Interestingly, the GAPS Diet excludes virtually all sources of dietary propionate and substrates for bacterial propionate production, which may at least in part explain the success of the diet for neurological conditions. After the initial elimination phase, the reintroduction of vegetables rich in inulin like broccoli and cauliflower may preferentially feed butyrate-producers.
The GAPS Diet is also ketogenic, particularly in the early stages. The effects of ketogenic diet on propionate metabolism is unknown, though propionate production is likely much lower due to carbohydrate restriction. Ketogenic diets also appear to increase overall NADH production and flux through the right side of the TCA cycle,52 potentially ameliorating some of the left-sidedness caused by excess propionate. Lastly, ketones use the same transporters as propionate and other SCFAs, and therefore may reduce the amount of propionate building up in cells by competing for transport.53
Takeaways
Okay, so we’ve covered quite a bit of ground. Clearly, there’s still a lot of progress that can be made to elucidate propionate’s connection to autism and related conditions, but let’s review the major takeaways:
- Autism and other neurological disorders are increasingly prevalent, and this cannot be explained by genetic influences
- Propionate has been most studied in autism, but it likely has implications for other neurological and mitochondrial-related diseases, too. Even someone suffering from brain fog or fatigue could potentially be experiencing the effects of excess propionate.
- Propionate can induce ASD-like behaviors in animal models. Human ASD patients have increased abundance of propionate-producing bacteria and elevated fecal propionate levels
- Propionate is not the only cause of autism, but it likely plays a major role in a subset of ASD patients. Propionate induces gap junction dysfunction, mitochondrial dysfunction, and carnitine dysfunction, particularly in the context of inflammation and acidosis.
- Antibiotics can transiently knock down propionate-producers but aren’t a long-term solution
- Supplementation with acetyl-L-carnitine, biotin, methyl-B12, and/or butyrate may be helpful
- The widespread use of propionate in food products is concerning. Removing wheat, dairy, and other dietary sources of propionate is likely to improve symptoms. Infant formula, refined carbohydrates, and artificial sweeteners stimulate the growth of propionate-producing bacteria.
- The GAPS Diet may help regulate propionate production by removing all fermentable carbohydrates for a period of time. Afterwards, strategic re-introduction of the right fibers may be able to stimulate the growth of beneficial butyrate-producers and keep propionate-producers at bay.
That’s all for now! Be sure to subscribe below so you don’t miss a post! Next up in the series: why I don’t trust fecal SCFA measurements.
Sources:
- DiCicco-Bloom, E. et al. The developmental neurobiology of autism spectrum disorder. J. Neurosci. Off. J. Soc. Neurosci. 26, 6897–6906 (2006).
- Chauhan, A. & Chauhan, V. Oxidative stress in autism. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 13, 171–181 (2006).
- James, S. J. et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 23, 2374–2383 (2009).
- Williams, B. L. et al. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLOS ONE 6, e24585 (2011).
- Skokauskas, N. & Gallagher, L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology 43, 8–16 (2010).
- Consortium, T. A. G. P. et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 39, ng1985 (2007).
- Barnevik-Olsson, M., Gillberg, C. & Fernell, E. Prevalence of autism in children born to Somali parents living in Sweden: a brief report. Dev. Med. Child Neurol. 50, 598–601 (2008).
- Mcmanus, C. M., Michel, K. E., Simon, D. M. & Washabau, R. J. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am. J. Vet. Res. 63, 295–300 (2002).
- Burger-van Paassen, N. et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 420, 211–219 (2009).
- Hatayama, H., Iwashita, J., Kuwajima, A. & Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 356, 599–603 (2007).
- Mortensen, F. V., Nielsen, H., Mulvany, M. J. & Hessov, I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31, 1391–1394 (1990).
- Mitsui, R., Ono, S., Karaki, S. & Kuwahara, A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 17, 585–594 (2005).
- MacFabe, D. F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 23, (2012).
- Chen, W.-J. L., Anderson, J. W. & Jennings, D. Propionate May Mediate the Hypocholesterolemic Effects of Certain Soluble Plant Fibers in Cholesterol-Fed Rats. Proc. Soc. Exp. Biol. Med. 175, 215–218 (1984).
- Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
- Lin, H. V. et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLOS ONE 7, e35240 (2012).
- Asquith, M. et al. Intestinal Metabolites Are Profoundly Altered in the Context of HLA-B27 Expression and Functionally Modulate Disease in a Rat Model of Spondyloarthritis. Arthritis Rheumatol. Hoboken NJ 69, 1984–1995 (2017).
- Feliz, B., Witt, D. R. & Harris, B. T. Propionic acidemia: a neuropathology case report and review of prior cases. Arch. Pathol. Lab. Med. 127, e325-328 (2003).
- Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 22, 512–519, e114-115 (2010).
- MacFabe, D. F. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 26, 28177 (2015).
- MacFabe, D. F. et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 176, 149–169 (2007).
- Foley, K. A., Ossenkopp, K.-P., Kavaliers, M. & MacFabe, D. F. Pre- and Neonatal Exposure to Lipopolysaccharide or the Enteric Metabolite, Propionic Acid, Alters Development and Behavior in Adolescent Rats in a Sexually Dimorphic Manner. PLOS ONE 9, e87072 (2014).
- Niehus, R. & Lord, C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. JDBP 27, S120-127 (2006).
- Wang, L. et al. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102 (2012).
- Finegold, S. M. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 17, 367–368 (2011).
- Shaw, W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 13, 135–143 (2010).
- Sandler, R. H. et al. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child Neurol. 15, 429–435 (2000).
- Rörig, B., Klausa, G. & Sutor, B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones. J. Physiol. 490, 31–49 (1996).
- Wiencken-Barger, A. E., Djukic, B., Casper, K. B. & McCarthy, K. D. A role for Connexin43 during neurodevelopment. Glia 55, 675–686 (2007).
- Rossignol, D. A. & Frye, R. E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry 17, 290–314 (2012).
- Frye, R. E. et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl. Psychiatry 6, e927 (2016).
- Karuri, A. R., Dobrowsky, E. & Tannock, I. F. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br. J. Cancer 68, 1080–1087 (1993).
- Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U. S. A. 101, 15718–15723 (2004).
- Jones, L. L., McDonald, D. A. & Borum, P. R. Acylcarnitines: role in brain. Prog. Lipid Res. 49, 61–75 (2010).
- Filipek, P. A., Juranek, J., Nguyen, M. T., Cummings, C. & Gargus, J. J. Relative carnitine deficiency in autism. J. Autism Dev. Disord. 34, 615–623 (2004).
- Pochini, L. et al. Interaction of beta-lactam antibiotics with the mitochondrial carnitine/acylcarnitine transporter. Chem. Biol. Interact. 173, 187–194 (2008).
- de Magistris, L. et al. Alterations of the Intestinal Barrier in Patients With Autism Spectrum Disorders and in Their First-degree Relatives. J. Pediatr. Gastroenterol. Nutr. 51, 418–424 (2010).
- Mellon, A. F., Deshpande, S. A., Mathers, J. C. & Bartlett, K. Effect of oral antibiotics on intestinal production of propionic acid. Arch. Dis. Child. 82, 169–172 (2000).
- Walker, A. W., Duncan, S. H., McWilliam Leitch, E. C., Child, M. W. & Flint, H. J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71, 3692–3700 (2005).
- Takuma, K. et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol. Biochem. Behav. 126, 43–49 (2014).
- Adams, J. B. et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 8, 34 (2011).
- Adams, J. B. et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 11, 111 (2011).
- Scotter, M. J., Thorpe, S. A., Reynolds, S. L., Wilson, L. A. & Strutt, P. R. Survey of baked goods for propionic acid and propionates. Food Addit. Contam. 13, 133–139 (1996).
- Lind, H., Jonsson, H. & Schnürer, J. Antifungal effect of dairy propionibacteria—contribution of organic acids. Int. J. Food Microbiol. 98, 157–165 (2005).
- Reichardt, N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8, ismej201414 (2014).
- Van den Abbeele, P. et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 13, 2667–2680 (2011).
- Al-Lahham, S. H., Peppelenbosch, M. P., Roelofsen, H., Vonk, R. J. & Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 1801, 1175–1183 (2010).
- Brouillet, E., Jacquard, C., Bizat, N. & Blum, D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 95, 1521–1540 (2005).
- Palmnäs, M. S. A. et al. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLOS ONE 9, e109841 (2014).
- Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
- Knol, J. et al. Colon Microflora in Infants Fed Formula with Galacto- and Fructo-Oligosaccharides: More Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 40, 36–42 (2005).
- Napoli, E., Dueñas, N. & Giulivi, C. Potential Therapeutic Use of the Ketogenic Diet in Autism Spectrum Disorders. Front. Pediatr. 2, (2014).
- Moschen, I., Bröer, A., Galić, S., Lang, F. & Bröer, S. Significance of Short Chain Fatty Acid Transport by Members of the Monocarboxylate Transporter Family (MCT). Neurochem. Res. 37, 2562–2568 (2012).
SCFAs Part 5: The propionate-autism connection
The prevalence of autism and other neuropsychiatric diseases has grown tremendously in recent decades. Environmental factors are sure to play a role, including the gut microbiota and its all-too important metabolites, short-chain fatty acids (SCFAs).
So far in the SCFA series, I’ve discussed the benefits of butyrate, the harms of excess butyrate, and the role of acetate in metabolic syndrome and obesity. In this article, I’ll talk about propionate, and its emerging connections to autism and other mitochondrial-related, neurological, and neurodevelopmental diseases. Don’t know what a SCFA is? Be sure to read my brief intro to SCFAs first!
The dramatic rise in autism spectrum disorders
When autism was first reported in the mid-20th century, it affected 1 in 10,000 people. Today, autism spectrum disorder (ASD) affects 1 in 68 people in the United States. ASD is characterized by social and communication impairments, sensory abnormalities, and repetitive behavior.1
Yet evidence increasingly suggests that ASD is not a brain disorder, but a “whole body” disorder. Those with ASD present with systemic abnormalities in metabolic and immune function,2,3 gastrointestinal symptoms, restrictive eating, and seizure disorders.4,5
Genes involved in mitochondrial function, immune regulation, and neural circuit formation have been linked to autism,6 but known genetic factors only account for 10-20% of ASDs. Some children even develop normally before regressing into ASD. This has especially been observed in populations that have migrated from underdeveloped countries to developed ones.7 Every Somalian population that has emigrated to Western countries has noticed an increased prevalence of ASD in their community. They call it the “Western Disease” since they don’t acquire it in their homeland of East Africa.
This means that environmental factors play a major role, and that autism arises from the complex interaction of genes with the environment. Recent evidence suggests that gut microbial metabolites, and, in particular, the SCFA propionate, may play a role. But before we dive into propionate in autism, let’s cover the roles that propionate plays in the body.
Too much of a good SCFA?
In part 3 of the SCFA series, we saw how four-carbon butyrate, a potent anti-inflammatory and crucial molecule for maintaining gut barrier function, could potentially be harmful in high concentrations, especially in the context of mucosal inflammation. Interestingly, a similar paradox may hold true for three-carbon propionate, where low concentrations have benefits, but high concentrations are toxic.
Propionate has wide-reaching effects on physiology. In the gut, propionate stimulates smooth muscle contractions,8 increases mucus secretion,9,10 promotes antimicrobial peptide expression, dilates colonic arteries11, and increases release of serotonin from gut endocrine cells.12 Propionate also influences intracellular pH, mitochondrial function, lipid metabolism, neurotransmitter synthesis and release, immune function, and gene expression.13 Propionate has been suggested to have weight loss, anti-inflammatory and cholesterol-lowering properties.14,15 In mice, propionate has been shown to induce the production of satiety hormones in the gut to reduce food intake and protect against diet-induced obesity16,17. A recent study found that administration of the short-chain fatty acid propionate significantly attenuated spondyloarthritis, a HLA-B27-associated inflammatory disease.17
However, excess propionate can be problematic. In propionic acidemia, a genetic error of metabolism, accumulation of propionate is associated with acidosis, developmental delay, seizure, increased oxidative stress, mitochondrial dysfunction, and bouts of gastrointestinal symptoms.18 Excess propionate has also been reported in irritable bowel syndrome19, and of course, autism.
A link between propionate and autism: animal models
Researcher Dr. Derek MacFabe is at the forefront of microbiome-related research and is largely responsible for elucidating the propionate-autism connection. His lab discovered that excess propionate is capable of inducing “behavioral and brain effects remarkably consistent with findings in persons with ASD”.20
In one study, they injected propionate directly into the cerebrospinal fluid of the rat’s brains. Within just 2-30 minutes, propionate-treated rats showed repetitive behaviors, increased hyperactivity, impaired social behavior, and evidence of seizure activity, compared to those receiving placebo infusion. While butyrate- and acetate-treated rats did show some abnormalities, propionate elicited the strongest ASD-like behaviors.21
In another study, they looked at the effects of systemic propionate by peripherally injecting pregnant rats and then their offspring. Propionate increased anxiety-like behavior and repetitive behavior, particularly in rats that were exposed both pre- and postnatally.22
Evidence for a propionate-autism link in humans
This link is potential, but still unproven in humans. As we saw with acetate, we can’t take be sure that what occurs in animal models also occurs in humans. In a recent review paper, Dr. MacFabe cautioned:
“The evidence of many effects of [propionate] on diverse biological pathways being consistent with findings with ASD patients is intriguing but largely correlative. Novel translational models […] coupled with longitudinal human studies are necessary to correlate these biomarkers to autistic regression or clinical improvement.” 20
We also don’t know whether an altered gut microbiota is the cause of ASD-related symptoms, or simply a consequence of the same underlying pathophysiological features of autism.
Still, it’s well accepted that birth by Cesarean section, early infections, and antibiotic exposure all may alter the developing gut microbiota, and are risk factors for ASD.23 Moreover, children with ASD have also been shown to have increased levels of fecal propionate,24 and ASD-associated bacteria include several known propionate-producing bacteria: Clostridia, Bacteroides, and Desulfovibrio.25
Several recent clinical studies have also found elevated urinary markers of Clostridia metabolites and altered carbohydrate metabolism in GI-tract biopsies in ASD patients,26 and others have reported temporary behavioral improvements following treatment with antibiotics that knockdown levels of propionate-producing bacteria.27
Despite the need for more human studies, the mechanisms at play in animal models also seem plausible in humans. We’ll dive into those in the next section.
The mechanisms: how propionate may contribute to autism
Fair warning: this section is full of biochemistry and complicated terminology. It’s not crucial, so if you get bogged down in the details, I’ll provide a summary at the end of the article.
Gap junction function
Dr. MacFabe postulates that many of the effects of propionate may be due to its ability to close gap junctions.28 Gap junctions connect the cytoplasm of adjacent cells and allow the passage of small molecules and ions between them. They are vital for synchronizing neural electrical activity, and play crucial roles in early brain development. A decrease in gap junction coupling may also inhibit cortical pruning, a phenomenon consistent with the increased density of neurons found in ASD patients.21
Furthermore, gap junction “knockout” mice (mice are genetically engineered to not express any gap junction proteins), have abnormal brain development and behaviors, seizure disorders, and exaggerated responses to neurotoxic insults.29
Mitochondrial dysfunction
ASD often occurs with genetic and biochemical changes that are consistent with mitochondrial dysfunction.30 Evidence suggests that propionate may be responsible for these changes by interfering with the mitochondrial TCA cycle.
The mitochondrial tricarboxylic acid (TCA) cycle is a key step in cellular energy production. The products of the TCA cycle are NADH and FADH2, which carry high energy electrons to the electron transport chain (ETC), where their reducing power is used to produce ATP (cellular energy). You can see from the diagram below that one round of the TCA cycle produces 3 molecules of NADH and 1 molecule of FADH2. Propionate enters the TCA cycle via conversion to succinyl CoA. In small amounts, this helps to maintain TCA cycle intermediates, and is beneficial to cellular energy production.

However, substantial amounts of propionate entering the TCA cycle bypasses the first four TCA enzymes and may cause a shift in the cycle. Flux increases through the latter half of the cycle, producing a buildup of citrate, while the proximal half becomes “backed up” due to feedback inhibition by succinyl CoA.

This has several consequences. First, this alters the ratio of NADH: FADH2, resulting in 1:1 production instead of 3:1. When these energy carriers reach the electron transport chain, NADH feeds into ETC Complex I and FADH2 feeds into Complex II.

Each NADH molecule results in the production of 3 molecules of ATP, while each FADH2 molecule results in the production of 2 molecules of ATP. Thus, a propionate-induced shift in TCA cycle flux will result in less NADH production, and a deficiency of energy carriers at Complex I, leading to less overall ATP formation. Indeed, children with ASD have been shown to have a deficiency in ETC complex 1.
Secondly, the buildup of mitochondrial citrate will result in citrate being transported into the cell cytosol. Citrate inhibits phosphofructokinase, the key regulatory step in glycolysis. Citrate also increases the formation of malonyl CoA, which inhibits CPT-1, the transporter that shuttles fatty acids into the mitochondria. This effectively blocks fatty acid oxidation.
Context matters: paradoxical propionate
To learn more about propionate’s role in mitochondrial dysfunction, students in Dr. McFabe’s laboratory cultured immune cells from ASD and control patients with various concentrations of propionate with and without reactive oxygen species (ROS). Paradoxically, they found that propionate improved mitochondrial function if ROS were not present; however, it negatively impacted mitochondrial function in the presence of ROS. In other words: propionate “can have both beneficial and toxic effects on mitochondrial function, depending on concentration, exposure duration, and microenvironment”.31
As a weak acid, propionate uptake increases under conditions of intracellular acidification and can become more concentrated within cells.32 Ongoing work by Dr. Sydney Finegold has identified bacterial populations like Desulfovibrio, which produce propionic acid and hydrogen sulfide. The presence of hydrogen sulfide may increase propionate’s ability to damage mitochondrial function33, possibly by acidifying cells.
The carnitine connection
Impairments in carnitine metabolism may also play a role in neurodevelopmental disorders such as ASD. Carnitine is most well-known for its involvement in fatty acid β-oxidation. Fatty acids cannot freely cross the mitochondrial membrane to be metabolized, and must instead be conjugated to the molecule carnitine for transport across the mitochondrial membrane. However, carnitines also play a role in lipid synthesis, cholinergic neurotransmission, membrane stability, and antioxidant activity. Carnitine is therefore important for cellular energy, brain development, and brain function.34
Many patients with ASD have a relative carnitine deficiency, which may be due to both inherited and acquired factors.35 For example, certain antibiotics are known to reduce carnitine levels.36 Oral carnitine and its derivative acetyl-L-carnitine have been shown to be neuroprotective and have promise as therapeutic agents in ASD and other neurodevelopmental disorders.
An epithelial energy crisis
If carnitine is deficient and a buildup of citrate is preventing fatty acids from being transported into the cell, brain cells aren’t the only ones that will suffer. Gut epithelial cells rely on fatty acid oxidation of butyrate for 70% of their cellular energy, and metabolism of this SCFA helps maintain gut integrity. Without fatty acid oxidation, the gut will become permeable.
Indeed, 36% of autistic patients have measurably increased intestinal permeability, and almost half have gastrointestinal symptoms of some sort.37
Can we test for propionate?
Of course, parents will be asking, can we get our child tested for propionate? Yes and no. There are several tests that might point to excess propionate, many of which I’ve already mentioned in some capacity:
- Fecal SCFA testing: measures levels of the SCFA propionate in stool
- Fecal microbiota testing: measures potential propionate-producing bacterial genera in the gut microbiota (Clostridia, Bacteroidetes, etc.)
- Urine Organic Acids Test Clostridia metabolites
- Urine Organic Acids Test TCA cycle metabolites: assessment of the ratio of distal half TCA metabolites to proximal half metabolites.
However, each of these has limitations. Propionate has a habit of hiding inside cells, which makes it difficult to detect, even in patients with a metabolic crisis. However, if several of the markers above are present, you can be more confident that you are dealing with propionate-induced or propionate-exacerbated ASD.
Strategies for modulating propionate production
So, let’s say propionate is causing dysfunction and exacerbating symptoms. What can we do? While most treatments are still empirical and based on anecdotal evidence, there are several ways to potentially reduce gastrointestinal propionate production and cellular levels of propionate.
Antibiotics
Metronidazole and vancomycin have been used in patients with ASD with some success. These antibiotics have broad-spectrum activity against gram-positive bacteria, including propionate-producing members of the Clostridium genus. Of the two, vancomycin is believed to be safer, since under normal circumstances, oral vancomycin is not significantly absorbed into circulation, while metronidazole is systemically absorbed and may have adverse systemic side effects.38
However, the effects of either antibiotic on propionate production may be short-lived. A small, 8 week, partially blinded clinical trial found that vancomycin was temporarily effective for treating ASD in 8 of 11 children, but the benefits didn’t last long.27 Clostridia quickly reappeared after cessation of antibiotic treatment, likely due to their spore-forming properties. Thus, antibiotics alone are not sufficient to knock down propionate production in the long-term.
Restoring gastrointestinal pH and SCFA ratios
Altering the pH of the gut has major consequences for gut microbiota composition. Studies of human fecal microbial communities found that at pH 5.5, beneficial butyrate-producing bacteria comprised 20% of the total population. When the pH rose to 6.5, these bacteria almost completely disappeared, and acetate- and propionate-producing bacteria became dominant.39 Of course, restoring colonic pH in a patient with ASD is no easy task, and is not a currently feasible treatment option.
On the other hand, butyrate has shown some promise for the treatment of ASD and other neurological disorders,40 though the mechanism is not completely understood. Of course, butyrate improves barrier function, regulates the immune system, and may help to reduce the pH in the gut, selecting against propionate-producers. Competition of butyrate for transporters may also reduce the amount of propionate absorbed from the gut into circulation. More research is necessary to determine how SCFA ratios may impact ASD.
Correcting nutrient deficiencies
Biotin and vitamin B12 are essential cofactors for the enzymes that break down propionate and allow it to enter the TCA cycle. Dietary deficiencies of these vitamins may further impair propionate and carnitine metabolism and contribute to mitochondrial dysfunction.

About half of biotin is produced by the gut microbiota, so those with gut dysbiosis are particularly susceptible to deficiency. One study found that children with ASD had significantly lower biotin levels compared to controls.41 A follow-up study found that replenishing biotin levels was highly correlated with ASD symptom improvement,42 suggesting that many ASD patients may benefit from biotin supplements.
Gluten-free, Casein-free Diet
In the last decade, gluten-free, casein-free (GFCF) diets have become an increasingly popular treatment for patients with ASD. While removing these immunogenic proteins from the diet may have independent benefits, GFCF diets may also have implications for propionate production.
Notably, propionate is a major animal silage and food preservative in wheat and dairy products as sodium propionate or calcium propionate salts, and occurs naturally in some dairy products like Swiss cheese.43,44
Propionate is also produced directly or indirectly by many ASD-associated bacteria, such as Clostridia and Desulfovibrio, from the fermentation of refined and wheat-based sugars.45,46 In other words, when you feed these bacteria certain grains and refined carbohydrates, they start making more propionate.
Removing these foods may have benefits on propionate metabolism and for gut barrier function. The same study mentioned above that found increased intestinal permeability in children with autism found that those on a GFCF diet had lower intestinal permeability scores than controls!37
Other dietary sources of propionate
Beyond wheat and dairy, propionate is also added to many refined foods as a preservative. In fact, the food industry and agriculture are increasingly using propionate and related chemical derivatives.47 Nitropropionate is a derivative of propionate produced by many plants and fungi. It’s a potential contaminant of sugar cane, soy sauce, and processed rice, and a potent mitochondrial and neurotoxin.48
Other foods stimulate propionate production. Artificial sweeteners like aspartame and saccharin, for example, has been shown to dramatically increase gut propionate levels in rodents.49,50 A 2005 study found that infants fed formula had 2.5 fold greater propionate levels than breastmilk-fed infants. Adding oligosaccharides to the formula only partially ameliorated the excess propionate production.51
The GAPS Diet and carbohydrates
Dr. Natasha Campbell-McBride is well known among the autism community for her book Gut and Psychology Syndrome (GAPS). Dr. McBride believes that neuropsychiatric disorders, including autism, arise in the gut. She has helped numerous children and adults reverse autistic behavior and regain normal daily function using a modified version of the Specific Carbohydrate Diet (SCD) called the GAPS Diet.
The GAPS Diet removes all fermentable carbohydrates from the diet for a period of time, allowing inflammation to subside and the gut barrier to heal and seal. Interestingly, the GAPS Diet excludes virtually all sources of dietary propionate and substrates for bacterial propionate production, which may at least in part explain the success of the diet for neurological conditions. After the initial elimination phase, the reintroduction of vegetables rich in inulin like broccoli and cauliflower may preferentially feed butyrate-producers.
The GAPS Diet is also ketogenic, particularly in the early stages. The effects of ketogenic diet on propionate metabolism is unknown, though propionate production is likely much lower due to carbohydrate restriction. Ketogenic diets also appear to increase overall NADH production and flux through the right side of the TCA cycle,52 potentially ameliorating some of the left-sidedness caused by excess propionate. Lastly, ketones use the same transporters as propionate and other SCFAs, and therefore may reduce the amount of propionate building up in cells by competing for transport.53
Takeaways
Okay, so we’ve covered quite a bit of ground. Clearly, there’s still a lot of progress that can be made to elucidate propionate’s connection to autism and related conditions, but let’s review the major takeaways:
- Autism and other neurological disorders are increasingly prevalent, and this cannot be explained by genetic influences
- Propionate has been most studied in autism, but it likely has implications for other neurological and mitochondrial-related diseases, too. Even someone suffering from brain fog or fatigue could potentially be experiencing the effects of excess propionate.
- Propionate can induce ASD-like behaviors in animal models. Human ASD patients have increased abundance of propionate-producing bacteria and elevated fecal propionate levels
- Propionate is not the only cause of autism, but it likely plays a major role in a subset of ASD patients. Propionate induces gap junction dysfunction, mitochondrial dysfunction, and carnitine dysfunction, particularly in the context of inflammation and acidosis.
- Antibiotics can transiently knock down propionate-producers but aren’t a long-term solution
- Supplementation with acetyl-L-carnitine, biotin, methyl-B12, and/or butyrate may be helpful
- The widespread use of propionate in food products is concerning. Removing wheat, dairy, and other dietary sources of propionate is likely to improve symptoms. Infant formula, refined carbohydrates, and artificial sweeteners stimulate the growth of propionate-producing bacteria.
- The GAPS Diet may help regulate propionate production by removing all fermentable carbohydrates for a period of time. Afterwards, strategic re-introduction of the right fibers may be able to stimulate the growth of beneficial butyrate-producers and keep propionate-producers at bay.
That’s all for now! Be sure to subscribe below so you don’t miss a post! Next up in the series: why I don’t trust fecal SCFA measurements.
Sources:
- DiCicco-Bloom, E. et al. The developmental neurobiology of autism spectrum disorder. J. Neurosci. Off. J. Soc. Neurosci. 26, 6897–6906 (2006).
- Chauhan, A. & Chauhan, V. Oxidative stress in autism. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 13, 171–181 (2006).
- James, S. J. et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 23, 2374–2383 (2009).
- Williams, B. L. et al. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLOS ONE 6, e24585 (2011).
- Skokauskas, N. & Gallagher, L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology 43, 8–16 (2010).
- Consortium, T. A. G. P. et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 39, ng1985 (2007).
- Barnevik-Olsson, M., Gillberg, C. & Fernell, E. Prevalence of autism in children born to Somali parents living in Sweden: a brief report. Dev. Med. Child Neurol. 50, 598–601 (2008).
- Mcmanus, C. M., Michel, K. E., Simon, D. M. & Washabau, R. J. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am. J. Vet. Res. 63, 295–300 (2002).
- Burger-van Paassen, N. et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 420, 211–219 (2009).
- Hatayama, H., Iwashita, J., Kuwajima, A. & Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 356, 599–603 (2007).
- Mortensen, F. V., Nielsen, H., Mulvany, M. J. & Hessov, I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31, 1391–1394 (1990).
- Mitsui, R., Ono, S., Karaki, S. & Kuwahara, A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 17, 585–594 (2005).
- MacFabe, D. F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 23, (2012).
- Chen, W.-J. L., Anderson, J. W. & Jennings, D. Propionate May Mediate the Hypocholesterolemic Effects of Certain Soluble Plant Fibers in Cholesterol-Fed Rats. Proc. Soc. Exp. Biol. Med. 175, 215–218 (1984).
- Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
- Lin, H. V. et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLOS ONE 7, e35240 (2012).
- Asquith, M. et al. Intestinal Metabolites Are Profoundly Altered in the Context of HLA-B27 Expression and Functionally Modulate Disease in a Rat Model of Spondyloarthritis. Arthritis Rheumatol. Hoboken NJ 69, 1984–1995 (2017).
- Feliz, B., Witt, D. R. & Harris, B. T. Propionic acidemia: a neuropathology case report and review of prior cases. Arch. Pathol. Lab. Med. 127, e325-328 (2003).
- Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 22, 512–519, e114-115 (2010).
- MacFabe, D. F. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 26, 28177 (2015).
- MacFabe, D. F. et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 176, 149–169 (2007).
- Foley, K. A., Ossenkopp, K.-P., Kavaliers, M. & MacFabe, D. F. Pre- and Neonatal Exposure to Lipopolysaccharide or the Enteric Metabolite, Propionic Acid, Alters Development and Behavior in Adolescent Rats in a Sexually Dimorphic Manner. PLOS ONE 9, e87072 (2014).
- Niehus, R. & Lord, C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. JDBP 27, S120-127 (2006).
- Wang, L. et al. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102 (2012).
- Finegold, S. M. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 17, 367–368 (2011).
- Shaw, W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 13, 135–143 (2010).
- Sandler, R. H. et al. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child Neurol. 15, 429–435 (2000).
- Rörig, B., Klausa, G. & Sutor, B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones. J. Physiol. 490, 31–49 (1996).
- Wiencken-Barger, A. E., Djukic, B., Casper, K. B. & McCarthy, K. D. A role for Connexin43 during neurodevelopment. Glia 55, 675–686 (2007).
- Rossignol, D. A. & Frye, R. E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry 17, 290–314 (2012).
- Frye, R. E. et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl. Psychiatry 6, e927 (2016).
- Karuri, A. R., Dobrowsky, E. & Tannock, I. F. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br. J. Cancer 68, 1080–1087 (1993).
- Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U. S. A. 101, 15718–15723 (2004).
- Jones, L. L., McDonald, D. A. & Borum, P. R. Acylcarnitines: role in brain. Prog. Lipid Res. 49, 61–75 (2010).
- Filipek, P. A., Juranek, J., Nguyen, M. T., Cummings, C. & Gargus, J. J. Relative carnitine deficiency in autism. J. Autism Dev. Disord. 34, 615–623 (2004).
- Pochini, L. et al. Interaction of beta-lactam antibiotics with the mitochondrial carnitine/acylcarnitine transporter. Chem. Biol. Interact. 173, 187–194 (2008).
- de Magistris, L. et al. Alterations of the Intestinal Barrier in Patients With Autism Spectrum Disorders and in Their First-degree Relatives. J. Pediatr. Gastroenterol. Nutr. 51, 418–424 (2010).
- Mellon, A. F., Deshpande, S. A., Mathers, J. C. & Bartlett, K. Effect of oral antibiotics on intestinal production of propionic acid. Arch. Dis. Child. 82, 169–172 (2000).
- Walker, A. W., Duncan, S. H., McWilliam Leitch, E. C., Child, M. W. & Flint, H. J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71, 3692–3700 (2005).
- Takuma, K. et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol. Biochem. Behav. 126, 43–49 (2014).
- Adams, J. B. et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 8, 34 (2011).
- Adams, J. B. et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 11, 111 (2011).
- Scotter, M. J., Thorpe, S. A., Reynolds, S. L., Wilson, L. A. & Strutt, P. R. Survey of baked goods for propionic acid and propionates. Food Addit. Contam. 13, 133–139 (1996).
- Lind, H., Jonsson, H. & Schnürer, J. Antifungal effect of dairy propionibacteria—contribution of organic acids. Int. J. Food Microbiol. 98, 157–165 (2005).
- Reichardt, N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8, ismej201414 (2014).
- Van den Abbeele, P. et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 13, 2667–2680 (2011).
- Al-Lahham, S. H., Peppelenbosch, M. P., Roelofsen, H., Vonk, R. J. & Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 1801, 1175–1183 (2010).
- Brouillet, E., Jacquard, C., Bizat, N. & Blum, D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 95, 1521–1540 (2005).
- Palmnäs, M. S. A. et al. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLOS ONE 9, e109841 (2014).
- Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
- Knol, J. et al. Colon Microflora in Infants Fed Formula with Galacto- and Fructo-Oligosaccharides: More Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 40, 36–42 (2005).
- Napoli, E., Dueñas, N. & Giulivi, C. Potential Therapeutic Use of the Ketogenic Diet in Autism Spectrum Disorders. Front. Pediatr. 2, (2014).
- Moschen, I., Bröer, A., Galić, S., Lang, F. & Bröer, S. Significance of Short Chain Fatty Acid Transport by Members of the Monocarboxylate Transporter Family (MCT). Neurochem. Res. 37, 2562–2568 (2012).

Fantastic articles Lucy! Thank you
regarding Propionate and Neurological dysfunction, you are probably aware that Sodium Propionate 500mg twice a day as a supplement is used (mainly in Germany) to alleviate symptoms of Multiple Sclerosis!
https://www.sciencedirect.com/science/article/pii/S0092867420302129
https://www.frontiersin.org/articles/10.3389/fimmu.2021.676016/full
Fantastic articles Lucy!Thank you
regarding Propionate and Neurological dysfunction, you are probably aware that Sodium Propionate 500mg twice a day as a supplement is used (mainly in Germany) to alleviate symptoms of Multiple Sclerosis!
https://www.sciencedirect.com/science/article/pii/S0092867420302129
https://www.frontiersin.org/articles/10.3389/fimmu.2021.676016/full
I really enjoyed the Butyrate series! I too would be interested in the 6th article — “Why I don’t trust fecal SCFA measurements.”
Hi Lucy,
Wonderful information in these blog posts.
According to Jason Hawrelak, prebiotics like lactulose can acidify the colon and this particular one also supports F. prausnitizii. So I think there are certainly interventions like this that can be used in ASD.
I have an adult client that I feel has ASD-type behaviours and when I checked his stool test – he does have high propionate and also high levels of the species that make it. Along with low butyrate. Super interesting.
Alice
Hi Lucyyy! how great is this, OMG… Do you have a link to the next in the series?
“why I don’t trust fecal SCFA measurements”???
It would be nice if you would link the previous parts